Review Article- Asian Journal of Pharmaceutical Technology and Innovation(2018)
Experimental Epidemiology – A Review
Suvarna V Biradar1* and Divya S22Senior Lecturer, Department of Oral and Maxillofacial Pathology, Vydehi Institute of Dental Sciences and Research Center, Bangalore, India
Dr. Suvarna V Biradar, Senior Lecturer, Department of Public Health Dentistry, HKDET’S Dental College Hospital & Research Institute, Humnabad 585330. Bidar, Karnataka, India, Tel: +918970831926, Email: suvarna13gulbarga@gmail.com
Received: 10-Jan-2018 Accepted Date: Jan 18, 2018 ; Published: 20-Jan-2018
Abstract
There are different epidemiological methods such as Descriptive, Analytical and Experimental. Experimental research is a study in which an investigator manipulates or controls one or more independent variables and observes the effect on dependent variables. Types of experimental studies – Randomized controlled Trials and Non-randomized Controlled Trials. There are various steps involved in randomized controlled trials. There are types of randomized controlled trials such as clinical trials, preventive trials, risk factor trials, cessation experiments, trial of etiological agents, evaluation of health services and community Intervention Trials(CITs).Further, there are types of Non-Randomized Controlled trials. There are Concurrent parallel study design and Cross - over type of study designs. There are various advantages and disadvantages of experimental studies. There are guidelines to be followed when conducting RCTs such as CONSORT guidelines.
https://linktr.ee/marmaris1 https://www.divephotoguide.com/user/marmaris1/ https://artmight.com/user/profile/2408931 https://allmyfaves.com/marmaris1 https://www.fimfiction.net/user/628252/marmaris https://www.drupalgovcon.org/user/568666 https://www.roleplaygateway.com/member/marmaris1/ https://www.kickstarter.com/profile/825997479/about https://tapas.io/sam659667 https://seedandspark.com/user/marmaris1 https://starity.hu/profil/386591-marmaris1/ https://www.informationweek.com/profile.asp https://nootheme.com/forums/users/marmaris1/ https://app.roll20.net/users/12308972/marmaris1-m https://www.360cities.net/profile/sam659667 https://fileforum.com/profile/marmaris1 https://wordpress.org/support/users/marmaris1/ https://www.hebergementweb.org/members/marmaris1.539143/ https://forum.cs-cart.com/u/marmaris1/ https://www.tntxtruck.com/User-Profile/UserId/12208 https://calis.delfi.lv/blogs/posts/205779-linkler/lietotajs/310821-marmaris1/ https://profile.ameba.jp/ameba/marmaris1/ https://www.avianwaves.com/User-Profile/userId/184770 https://engine.eatsleepride.com/rider/marmaris1 https://keymander.iogear.com/profile/53051/marmaris1 https://www.mifare.net/support/forum/users/marmaris1/ https://inkbunny.net/marmaris1?&success=Profile+settings+saved. https://www.diggerslist.com/64e0c4fad170b/about https://research.openhumans.org/member/me/ http://bluerevolutioncrowdfunding.crowdfundhq.com/users/marmaris1 https://www.cakeresume.com/me/marmaris1 https://educatorpages.com/site/marrmaris1/pages/about-me? https://gotartwork.com/Profile/marmaris1-marmaris1/253385/ https://www.facer.io/user/OGfo7VWrhR https://able2know.org/user/marmaris1/ https://www.techrum.vn/members/marmaris1.230749/#about http://foxsheets.statfoxsports.com/UserProfile/tabid/57/userId/145739/Default.aspx http://riosabeloco.com/User-Profile/userId/196075 http://phillipsservices.net/UserProfile/tabid/43/userId/245691/Default.aspx http://www.ramsa.ma/UserProfile/tabid/42/userId/962725/Default.aspx http://krachelart.com/UserProfile/tabid/43/userId/1242308/Default.aspx http://kedcorp.org/UserProfile/tabid/42/userId/72907/Default.aspx http://atlantabackflowtesting.com/UserProfile/tabid/43/userId/561626/Default.aspx https://www.intensedebate.com/people/marmaris12 https://rosalind.info/users/marmaris1/ https://wordpress.com/me https://photozou.jp/user/top/3342211
Keywords
Epidemiology, Randomized controlled trials, CONSORT GuidelinesIntroduction
The word Epidemiology is derived from the Greek term – Epidemic where
EPI – UPON
DEMOS – PEOPLE
LOGOS – STUDY OR SCIENCE
PARKIN (1873)
“The branch of medical sciences which deals with the treatment of epidemics”
(Source : Soben Peter Essentials of Preventive and Community Dentistry 4th edition pg.no.42-80)
MACMOHAN (1960)
“The study of the distribution and determinants of disease frequency in man”
(Source : Soben Peter Essentials of Preventive and Community Dentistry 4th edition pg.no.42-80)
JOHN M LAST (1988)
“The study of the distribution and determinants of health related states or events in specified populations and the application of this study to the control of health problems”
(Source : K Park’ s Textbook of Preventive and Social Medicine 21st edition pg.no. 76-82)
EPIDEMIOLOGICAL METHODS
There are different epidemiologic methods such as :
I. DESCRIPTIVE EPIDEMIOLOGY
II. ANALYTICAL EPIDEMIOLOGY
III. EXPERIMENTAL EPIDEMIOLOGY
UNIQUE FEATURES OF EXPERIMENT
❏ Experiments provide the strongest basis for inferring causal relationships
❏ When an experiment is conducted the researcher's interest is always in determining the relationship between Cause and Effect (Causal relationship)
CLASSIFICATION OF EXPERIMENTS
Experiments have been classified as :
I. Laboratory experiments
II. Animal experiments
III. Human experiments
I. LABORATORY EXPERIMENTS
Advantages
1. It allows for precise control of variables
2. Experiments can be replicated. We cannot generalize from the results of a single experiment. The more often an experiment is repeated, with the same results obtained, the more confident we can be that the theory being tested is valid.
Disadvantage
Artificiality
The experiment is not typical of real life situations. It will be difficult to generalise findings from experiments because they are not ecologically valid (true to real life).
II. ANIMAL EXPERIMENTS
PURPOSES
1. Experimental reproduction of human disease in animals to confirm aetiological hypothesis
2. To test the efficacy of various therapeutic and preventive measures such as vaccines and drugs
3. To study the natural history of disease
4. Animal experiments are done on carefully bred animals in controlled environments. Animals are bred in laboratories and manipulated easily according to wishes of the experimenter
5. They multiply rapidly and enable experimenter to carry out certain experiments which are not possible in human beings
LIMITATIONS
1. All human diseases cannot be reproduced in animals
2. Results of animal studies cannot be extrapolated to human beings
III. HUMAN EXPERIMENTS
1. To investigate disease etiology
2. To evaluate the preventive and therapeutic measures
Examples of human experiments are :
James Lind (1747) – Study done on group of soldiers to reveal the association of lemons, oranges in diet on scurvy.
Edward Jenners (1796) experimental work with Cowpox to develop vaccine against Small pox
❏ Human studies should be carefully designed because they involve logistic and ethical implications.
Aims of experimental epidemiology
❏ To provide “Scientific proof” of etiological or risk factors which may permit the modification or control of those diseases
❏ To provide a method of measuring the effectiveness and efficiency of health services for the prevention, control and treatment of disease and improve the heath of the community
Features of experimental epidemiology
1. The experiment is observed and the outcome is compared between experimental and control groups to statistically know the association between independent and dependent variables
2. Experimental research allows causal effects to be tested
3. It can be of predictive values where the expected results of certain interventions can be predicted precisely
4. Investigator should identify independent and dependent variable at an early stage
Independent variable is that which we are testing and dependent variable is the outcome PRINCIPLES OF EXPERIMENTAL EPIDEMIOLOGY
There are different principles of epidemiology. They are :
I. The principle of Replication
II. The principle of Randomization
III. The principle of Local Control
I. The principle of Replication
Replication refers to repeatability of the experiment in a similar setting.The purpose is to increase THE PRECISION OF RESULTS and to obtain a better estimate of the experimental errors. It facilitates other researchers to duplicate the study and check whether the results obtained are similar or dissimilar.
II. The principle of Randomization
Randomization is the technique whereby each subject in the research has an equal probability of being included in either test group or control group
❏ It is a statistical technique of assigning subjects into different groups randomly (free of preferences)
❏ Randomization makes two groups equivalent with respect to several variables (age, gender, diet) thus it ensures baseline comparability between the groups.
III. The principle of Local Control
According to this principle – we first divide the field into several homogenous parts known as BLOCKS and then each block is divided into parts equal to the number of treatments. Then, the treatments are randomly assigned to these parts of a block. Dividing the field into several homogenous parts is known as BLOCKING. The extraneous factor – the known source of variability is made to vary deliberately in such a way that the variability it causes can be measured and hence eliminated from the experimental error.
V. Classification of Experimental trials
Experimental trials are classified as Randomised Controlled Trials and Non Randomised controlled trials. Further, Randomised controlled trials are classified as Randomised Controlled Clinical Trials and Randomised Controlled Field Trials.
(Source - James F Jekel, David L Katz Epidemiology Biostatistics and Preventive Medicine 3rd edition 2007 pg.no. 83- 86).
Experimental designs have been classified as Informal and Formal Experimental Designs.Further, Informal Experimental Designs are classified as follows :
1. Before and after without control design
2. After – only with control design
3. Before and after with control design
Formal experimental designs are classified as :
1. Completely Randomized Design (C.R.design)
2. Randomized Block Design (R.B.Design)
3. Latin Square Design (L.S.design)
4. Factorial designs
INFORMAL EXPERIMENTAL DESIGNS
1. Before and after without control design
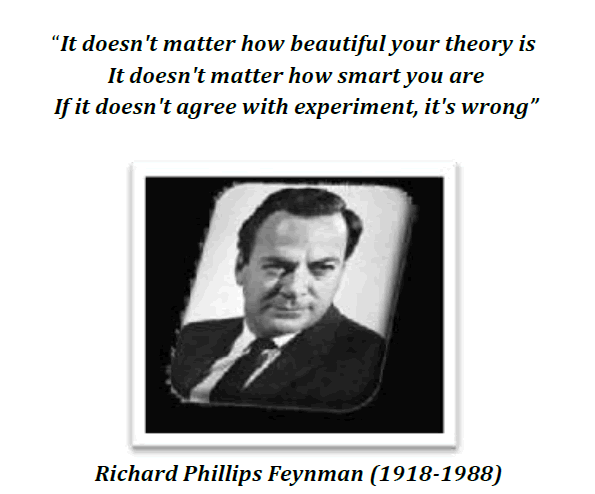
In such a design a single test group or area is selected and dependent variable is measured before the introduction of the treatment. The treatment is then introduced and the dependent variable is measured again. The effect of the treatment would be equal to the level of the phenomenon after the treatment minus the level of the phenomenon before the treatment. The design can be represented as follows :
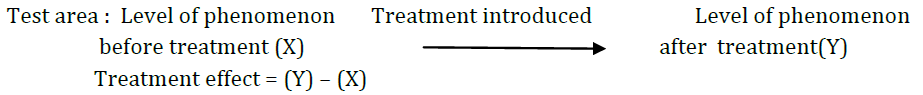
2. After-only with control design
this design, two groups or areas (test area and control area) are selected and the treatment is introduced into the test area only. The dependent variable is then measured in both the areas at the same time. Treatment impact is assessed by substracting the value of the dependent variable in the control area from its value in the test area. The design can be represented as follows :
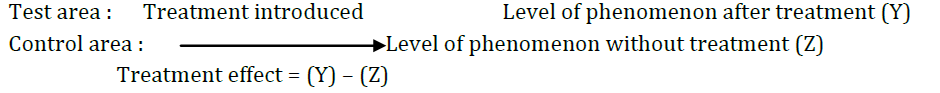
3. Before and after with control design
In this design, two areas are selected and the dependent variable is measured in both the areas for an identical time period before the treatment.The treatment is then introduced into test area only, and the dependent variable is measured in both for an identical time period after the introduction of the treatment.The treatment effect is determined by substracting the change in the dependent variable in the control area from the change in the dependent area in test area.This design is as shown below :
FORMAL EXPERIMENTAL DESIGNS
1. Completely randomized design (C.R.Design)
This design involves two principles. They are the principle of replication and the principle of randomization. It is the simplest possible design and its procedure of analysis is also easier. For instance, if we have 10 subjects and if we wish to test five under treatment A and five under treatment B, the randomization process gives every possible group of five subjects selected from a set of ten, an equal opportunity of being assigned to treatment A and treatment B.
There are two forms of such a design. They are :
1. Two group simple randomized design
2. Random replications design
1. Two group simple randomized design
In this design, firstly from the population sample is selected randomly. The sample will be then assigned randomly into experimental and control groups. Thus, this design yields two groups as representatives of the population. The design is as shown below :
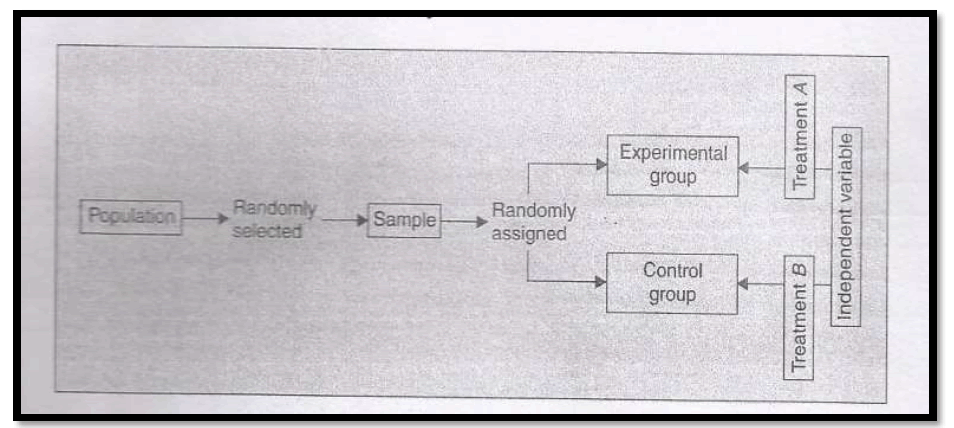
2. Random replications design
The random replication design serves two purposes.Firstly, it provides control for the differential effects of the extraneous independent variables and secondly, it randomizes any individual differences among those conducting the treatments. Diagrammatically, this design is illustrated as follows :
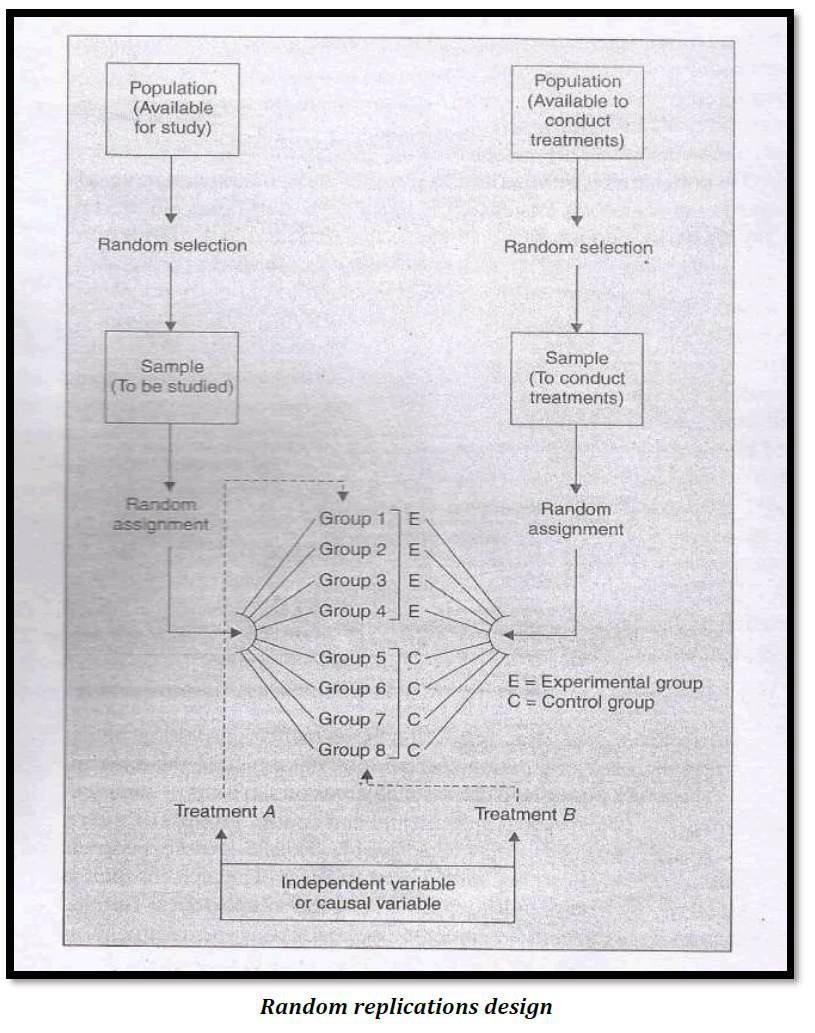
2. Randomized Block design (R.B.Design)
In this design, subjects are first divided into groups, known as blocks, such that within each group the subjects are relatively homogenous with respect to some selected variable. At The number of subjects in a given block would be equal to the number of treatments and one subject in each block would be randomly assigned to each treatment. Blocks are the levels at which we hold the extraneous factor fixed.
Suppose four different forms of a standardized test in statistics were given to each of five students (selected one from each of the five I.Q. blocks) and the following are the scores which they obtained.
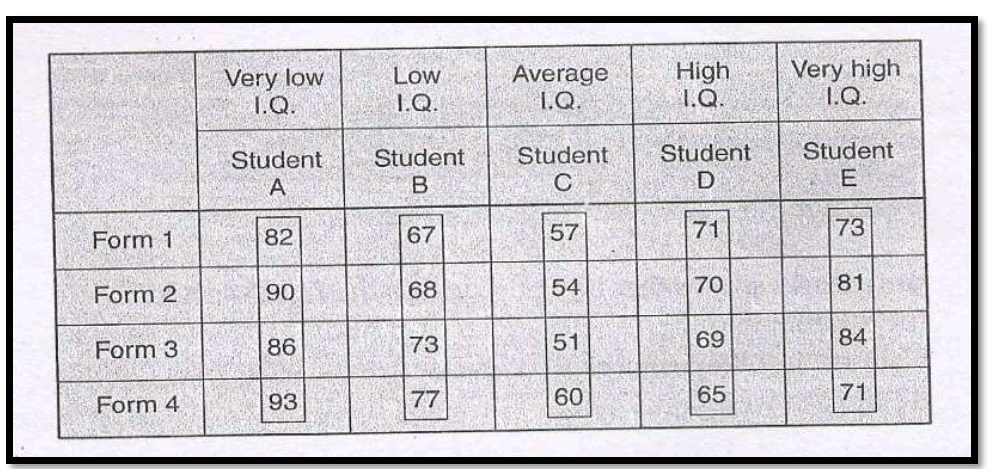
3.Latin Square Design (L.S.Design)
Latin square design is an experimental design which is frequently used in agricultural research.For instance, an experiment has to be made through which the effects of five different varieties of fertilizers on the yield of a certain crop, say wheat is to be judged. The Latin square design is one wherein each fertilizer appears five times but is used only once in each row and in each column of the design. The two blocking factors may be represented through rows and columns.
The following is a diagrammatic form of such a design say five types of fertilizers A,B,C,D and E and the two blocking factors such as soil fertility and the varying seeds :
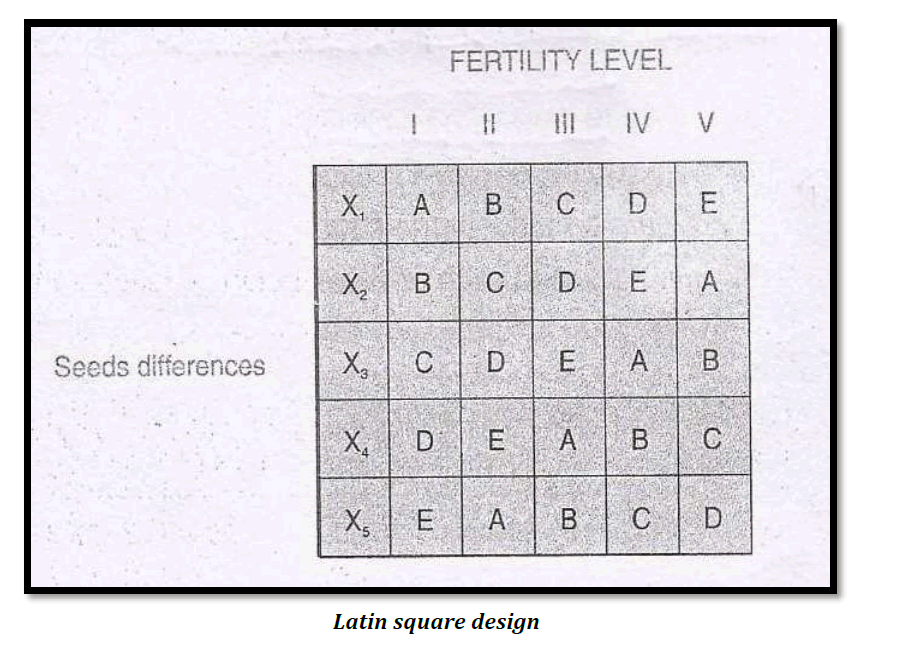
4. Factorial Designs
Factorial designs are used in experiments where the effects of varying more than one factor are to be determined. Factorial designs are of two types. They are :
1. Simple factorial designs
2. Complex factorial designs
1. Simple factorial designs
In this design, we consider the effects of varying two factors on the dependent variable. This design is also known as ‘two factor factorial design’.
Illustration
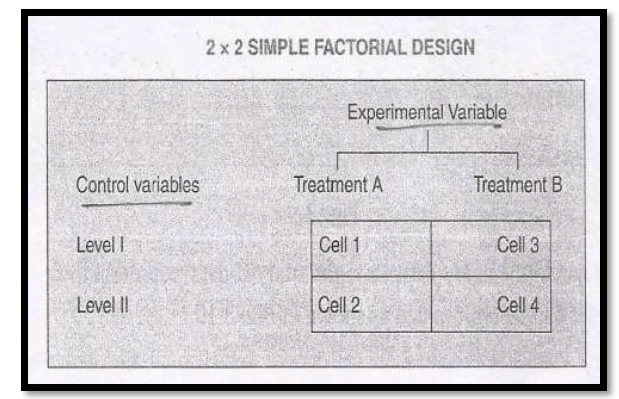
In this design, the extraneous variable to be controlled by homogeneity is called the control variable and the independent variable which is manipulated is called the experimental variable. Then, there are two treatments of the experimental variable and two levels of the control variable. As such there are four cells into which the sample is divided. Each of the four combinations would provide one treatment or experimental condition.
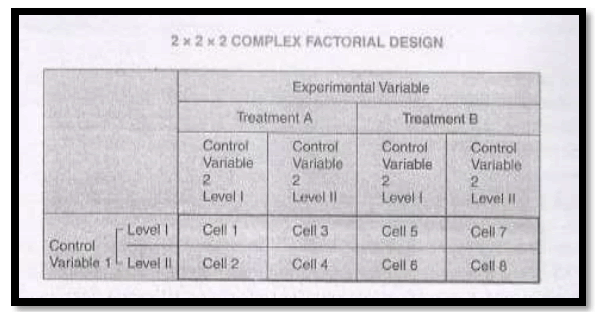
2. Complex factorial designs
A design which considers three or more independent variables simultaneously is called a complex factorial design.
In case of three factors with one experimental variable having two treatments and two control variables, each one of which having two levels, the design used will be termed as 2 X 2 X 2 complex factorial design which will contain a total of eight cells as shown in the following figure.
RANDOMISED CONTROLLED TRIAL
The design of the randomised controlled trial is as follows :
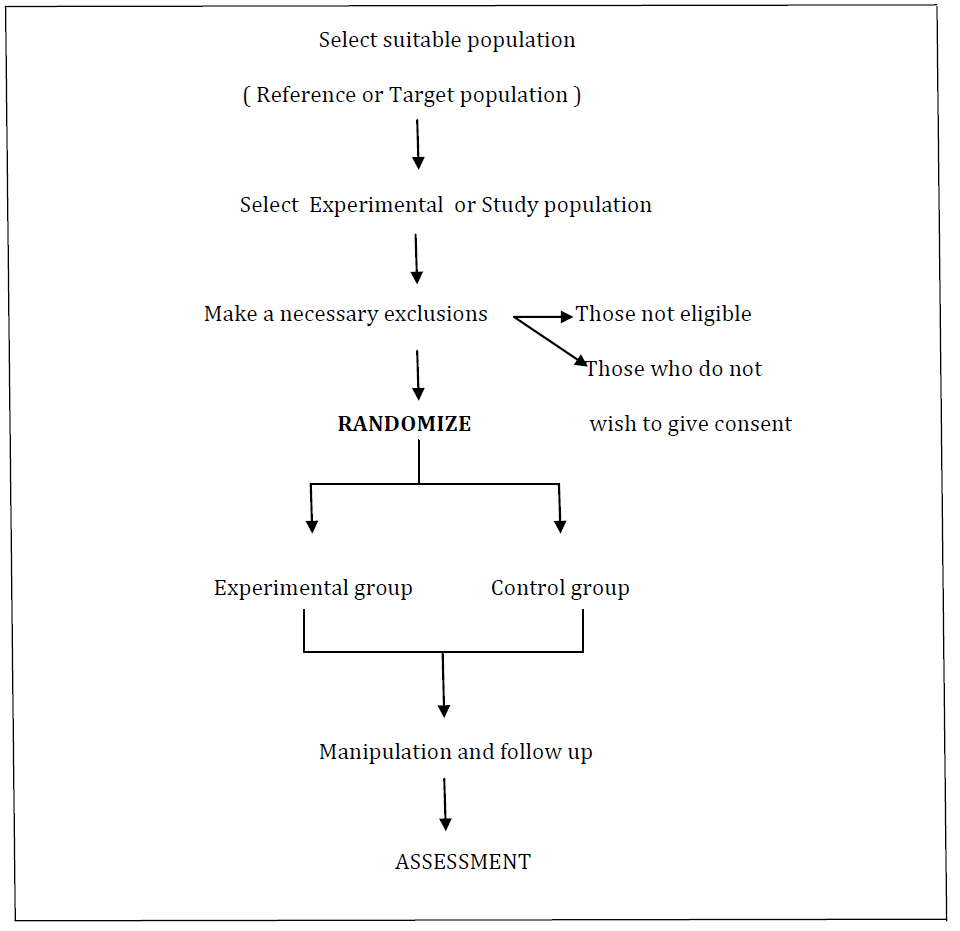
Steps involved in randomized controlled trials are as follows :
I. Drawing up a protocol
II. Selecting reference and experimental populations
III. Randomization
IV. Intervention or Manipulation
V. Follow up
VI. Assessment of outcome
I. Drawing up a protocol
Drawing up a protocol specifies the aims and objectives of the study, criteria for the selection of study and control groups, the sample size, the procedures for the allocation of subjects into the study and control groups, treatments to be applied and standardization of working procedures and schedules up to the stage of evaluation of outcome of the study.
II. Selecting reference and experimental populations
The experimental population is selected from the reference population. The results of experimental population if found successful, it is applied for the reference population.The subjects of the experimental population should provide the informed consent, should be the representatives of study population and qualified or eligible for the trial.
3. Randomization
Randomization is a statistical procedure by which the participants are allocated into groups usually called “Study” and “Control” groups, to receive or not to receive an experimental, preventive or therapeutic procedure or intervention.
Its an attempt to eliminate “bias” and allows for comparability. By random allocation every individual gets an equal chance of being allocated into either group.
TYPES OF RANDOMIZATION
1. Simple randomization
2. Restricted randomization
❏ Permuted-block randomization or blocked randomization
❏ Adaptive biased-coin randomization methods
3. Adaptive randomization
4. Stratified randomization
1. Simple randomization
This is a commonly used procedure, similar to "repeated fair coin-tossing." Also known as "complete" or "unrestricted"randomization. Its main drawback is the possibility of imbalanced group sizes in small RCTs. It is therefore recommended only for RCTs with over 200 subjects.
2. Restricted randomization
To balance group sizes in smaller RCTs "restricted" randomization is recommended.
A. Permuted-block randomization or blocked randomization
A "block size" and "allocation ratio" (number of subjects in one group versus the other group) are specified, and subjects are allocated randomly within each block. For example
A block size of 6 and an allocation ratio of 2:1 would lead to random assignment of 4 subjects to one group and 2 to the other.
❏Even if the block sizes are large and randomly varied, the procedure can lead to selection bias
❏ For the "proper" analysis of data from permuted-block-randomized RCTs requires stratification of blocks.
B. Adaptive biased-coin randomization methods
Uncommon methods, the probability of being assigned to a group decreases if the group is over-represented and increases if the group is under-represented. The methods are thought to be less affected by selection bias than permuted-block randomization.
3. Adaptive Randomization
Much less frequently used than simple or restricted randomization.
Response-adaptive randomization
Also known as outcome-adaptive randomization.The probability of being assigned to a group increases if the responses of the prior patients in the group were favourable.
4. Stratified randomization
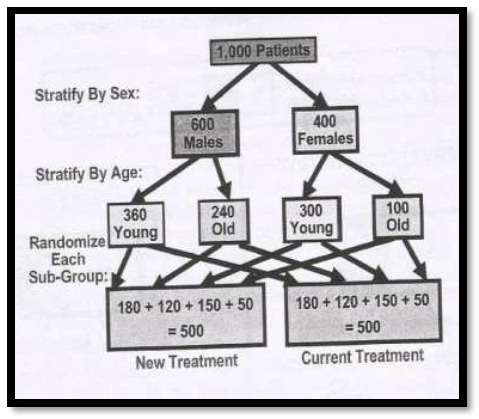
Example : There are 1,000 patients and firstly they are stratified into males and females based on sex followed by theres stratification by age as young and old. Further, each sub-group is randomly allocated in to two groups as – the group which receives the new treatment and the group which receives the current group as shown in the above presentation.
Suitable controls
The use of control treatments is essential for evaluation of benefits of a particular agent or product. The choice of controls differ depending on many aspects.
Types of control
• Bench mark control
• Positive control
• Negative control
• Placebo control
1. Bench mark control
This term is usually used to define a control which is a commercially available product.It is to find out whether the tested product is having superior efficacy than conventionally used product. Example – Listerine mouth wash.
2. Positive control
It is an agent presently considered to be the best and most effective available agent. Example – Hexidine mouth wash.
3. Negative control
In a trial assessing the effectiveness of 4% Tulsi mouth rinse against Streptococcus mutans, the control group is placed under distilled water which is a negative control.
4. Placebo control
Placebo control consists of a placebo (inert substance) administered and very similar to the test drug in order to counteract or balance the placebo effect induced by the test drug. Example – Gumbase without Xylitol.
Further, the types of controls include :
❏ No treatment concurrent control
❏Dose-response concurrent control
❏ External control (including historical control)
❏ Multiple control groups
❏ No treatment concurrent control – essentially same as placebo but unable to blind
❏ Dose response concurrent control – several variations with or without placebo
❏ Multiple control groups – gets complexed
❏ External control - A new treatment prospectively used in a series of patients preferably with same inclusion / exclusion criteria, same study procedures followed.
❏Compared with previous series of comparable patients, e.g., earlier study for same indication
Example : A clinical trial was conducted to assess the effect of a herbal chewing gum on Salivary mutans Streptococci among high schoolchildren in Davangere city.
In this study,
❏ Test intervention is herbal chewing gum
❏ Positive control is Xylitol chewing gum.
❏ Placebo control is gum base without Xylitol
4. Manipulation
The next step is to intervene or manipulate the experimental group by deliberate application or withdrawal or reduction of the suspected causal factor as laid down in the protocol.
5. Follow up
The experimental and control group subjects are examined at defined intervals of time, in a standard manner under the same given circumstances till the final assessment of outcome. Some losses to follow up are inevitabl and this is known as “ATTRITION”. These losses may be due to death, migration of participants and loss of interest.
6. Assessment
This is the final step of the trial. The outcome may be positive or negative. During the assessment of the outcome, bias is inevitable. The sources of bias are - bias on the part of participants, observer bias and the bias in evaluation.
TYPES OF RANDOMIZED CONTROLLED TRIALS
I. CLINICAL TRIALS
II. PREVENTIVE TRIALS
III. RISK FACTOR TRIALS
IV. CESSATION EXPERIMENTS
V. TRIAL OF ETIOLOGICAL AGENTS
VI. TRIAL OF ETIOLOGICAL AGENTS
VII. COMMUNITY INTERVENTION TRIALS (CITs)
I. CLINICAL TRIAL
It is an experimental design used by clinicians and epidemiologists to evaluate drugs or clinical procedures.It is also defined as a research strategy in which administration of a test regimen in humans is evaluated for its efficacy and safety.The most common form is the randomized, double-blind, controlled clinical trial.
TYPES OF CLINICAL TRIALS
A. PROPHYLACTIC TRIALS
• Example : Immunization
B. THERAPEUTIC TRIALS
• Example : Drug Treatment, Surgical procedure
C. SAFETY TRIALS
• Example : Side effects of Oral Contraceptives and injectables
D. RISK FACTOR TRIALS
• Example : Smoking
PHASES OF CLINICAL TRIALS
There are five phases of clinical trials. They are :
PHASE I CLINICAL TRIAL
PHASE II CLINICAL TRIAL
PHASE III CLINICAL TRIAL
PHASE IV CLINICAL TRIAL
PHASE V CLINICAL TRIAL
PHASE I CLINICAL TRIAL
Studies of volunteers who receive initially a fraction of what the anticipated dose of drug is likely to be.They are monitored for the effects on body functions such as hepatic, cardiovascular, renal, gastrointestinal and endocrine. Example : Amoxicillin is used for the treatment of dental conditions. Before it is marketed, it undergoes various phases of clinical trial.
PHASE II CLINICAL TRIAl
This phase of clinical trial is carried out on 100-200 patients.Volunteers are selected according to strict criteria.To assess the effectiveness of the drug or device. To determine the appropriate dosage of the drug.To investigate the safety of the drug.
PHASE III CLINICAL TRIAL
In this phase, the drug (Amoxillin) which is tested on a large group of population has to be approved by the regulatory agency such as FEDERATION OF FOOD AND DRUG ADMINISTRATION (FDA) and also be lincensed by their respective state governments before it is permitted for marketing.
Strict criteria for the inclusion and exclusion from the trial are followed. The purposes of this phase of clinical trial are to assess the effectiveness of the drug, to assess the safety of the drug in continued use in a larger and more heterogenous population than in phase II. It requires superior clinical and epidemiological skills. Planning, organization and strict adherence to the preformulated protocols and instructions especially in multi-centric collaborative trials is required.
PHASE IV CLINICAL TRIAL
The purpose of this trial is to re-assess effectiveness, safety, acceptability and continued use of the drugs or devices.Post marketing surveillance for evaluating long term adverse effects - carcinogenesis and teratogenesis of the drugs is done.
Recently - PHASE V CLINICAL TRIAL has been evaluated to compare effectiveness research and communitybased research. It is used to signify the integration of a new clinical treatment into widespread public health practice.
Example – When Amoxicillin is tested for its use for dental conditions, it has come to know that the same drug can be used for treating other conditions such as Acute Phargngitis.
II. PREVENTIVE TRIALS
The purpose is to prevent or eliminate disease on an experimental basis.
Example - Trials of VACCINES (Polio vaccine)
ANALYSIS OF PREVENTIVE TRIALS
The analysis of preventive trials will be done to know the benefit the community will derive from the measures taken, the risks involved and the cost of the health services in terms of money, men and resources.
III. RISK FACTOR TRIALS
They are also type of preventive trials. In these types of trials, the investigator INTERVENES to INTERRUPT the usual sequence in the development of the disease for those individuals who have “RISK FACTOR” for developing the disease.
Example : The risk factors for developing CORONARY HEART DISEASE are elevated blood cholesterol levels, hypertension, smoking and sedentary habits. To reduce the risk for developing the coronary heart disease, investigator intervenes by interrupting for the reduction of elevated blood cholesterol levels, control of hypertension, cessation of smoking and regular physical activity.
Risk factor trials are of two types. They are single factor trials and multifactor trials. Both the approaches are complementary and are needed.
IV. CESSATION EXPERIMENTS
The another type of preventive trials are the cessation experiments. In these type of trials an attempt is made to EVALUATE the TERMINATION of the habit or the disease. If such action is followed by a significant reduction in the disease, THE HYPOTHESIS OF CAUSE IS GREATLY STRENGTHENED.
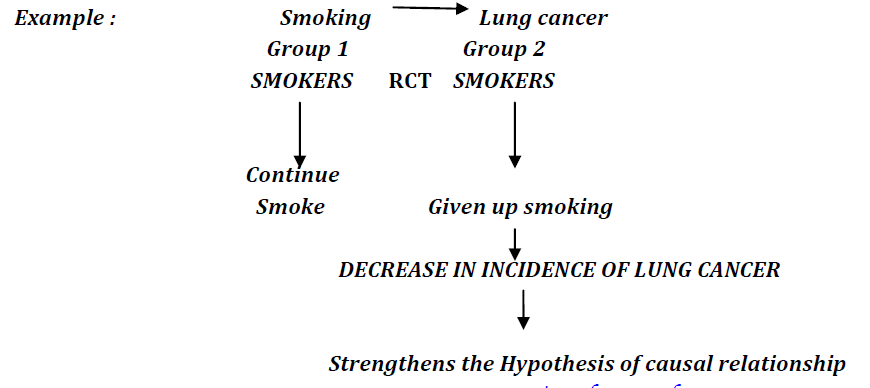
Smoking causes lung cancer. There are two groups. Group 1which consists of individuals who smoke and and group 2 which consists of individuals who do not smoke. Randomised clinical trial is conducted. After intervention, group 1 continues to smoke while group 2 quits smoking.As a result, group 2 shows decrease in the incidence of lung cancer. This strengthens the hypothesis of causal relationship.
V. TRIAL OF ETIOLOGICAL AGENTS
These trials helps to confirm or refute an etiological hypothesis.Since most diseases are fatal, disabling or unpleasant human experiments to confirm an etiological hypothesis are rarely possible.
VI. EVALUATION OF HEALTH SERVICES
RCTs helps to assess the effectiveness and efficiency of health services.Choices have to be made between alternative policies of health care delivery.Limited resources and priorities must be set for the implementation of large number of activities.
VII. COMMUNITY INTERVENTION TRIALS (CITs)
These are carried out in hospitals in patient groups with specific health conditions.The difference between RCT and CITs is that Randomization is done on communities in CITs rather than individuals.
Example : Testing a VACCINE for Small Pox
There are communities which receives VACCINE and other communities - will not be vaccinated OR will be vaccinated with a placebo.
CONTAMINATION AND CO-INTERVENTION become serious problems in CITs. Contamination occurs when individuals from one of the experimental groups receive the intervention from the other experimental group.
Example : There are two groups, one group which receives Iron fortified salt (group 1) and the other group receives non fortified salt(group 2). When individuals from group 2 might hear about iron fortified salt, some of them may acquire it from other community – group 1. This results in contamination.
Co-intervention occurs when other interventions, either unknown to investigators of this trial or are simultaneously introduced, in which case comparison of results from the two randomized groups will no longer be a reflection of the intervention under trial.
Most of the community intervention trials involve EVALUATIVE STRATEGIES TO STUDY COMMUNITY HEALTH SERVICES.
Examples : Community diagnosis
Design evaluation
Statistical significance Versus Clinical significance
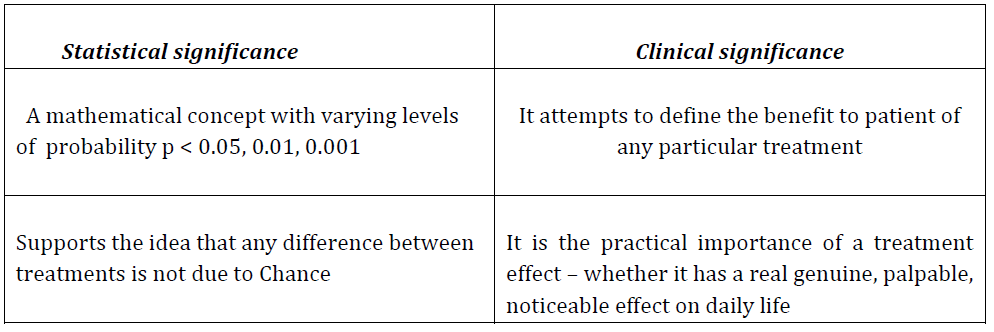
Clinical significance of the trial is represented by :
1. Effect size ( d )
2. Number Needed to Treat (NNT)
1. Effect size (d)
Effect size refers to the magnitude of effect of independent variable on the dependent variable. It is represented as “ Delta”. It is used to calculate clinical significance when the outcome variable is measured on a continuous scale.
Effect size interpretation
<0.2 is small
0.5 = Moderate effect
>0.8 = large effect
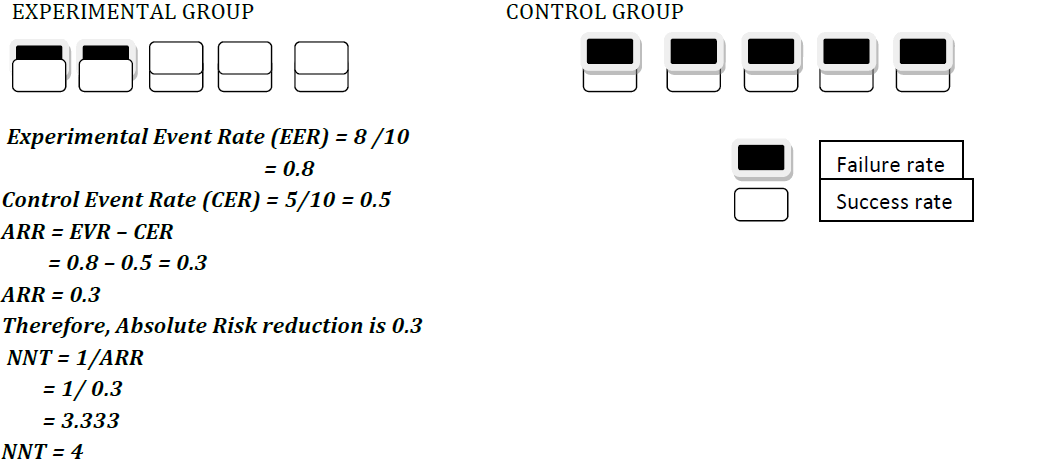
2. Number Needed to Treat (NNT)
NNT is the number of patients must be treated with the proposed therapy in order to have one additional successful results.
❏ NNT is the reciprocal of Absolute Risk Reduction (ARR)
❏ NNT of <10 is considered as clinically significant.
❏ It means for every ten patients treated at least one additional successful result is achieved
❏ NNT of >10 has very poor clinical significance
FORMULA FOR NNT
NNT = 1/ ARR
ARR = Experimental event rate (EER) - Control event rate (CER)
Calculation of ARR
EXPERIMENTAL GROUP CONTROL GROUP
Adjustment for dropouts
Let us say minimum sample size required for a study is 120, anticipated loss to follow up is 40%, what should be the sample size that we need to recruit?

= 1200/6
N1 = 200
100 eligible subjects in each group have to be approached and recruite Analysis strategies in community based intervention trials
There are many statistical procedures with an array of underlying assumptions that can be used to analyse the data arising from the randomized trials. These include :
I. Intent – to - treat approach
II. Modified intent – to treat analysis
I. Intent to treat approach
Is used for the primary analysis of data implying that all subjects will be counted in the group to which they have been assigned and that outcome data on all subjects will be included in the analysis.
II. Modified intent – to treat analysis
Certain subjects are removed on the grounds that treatment - related bias is highly unlikely while statistical power will be improved.
For example – in vaccine trials it is common to remove subjects who fail to receive even a single injection of the vaccine assigned.
Secondary analysis is performed that includes all subjects randomized.
Allocation concealment
Refers to procedures that secure strict implementation of the schedule of random assignments by preventing the foreknowledge of forthcoming allocations by study participants or by those recruiting them to the trial.
It is always feasible to conceal allocation. Failure to conceal allocation may lead to biased selection of participants into intervention groups.
Examples of procedures usually considered adequate includes :
1. Sequentially numbered drug containers of identical appearance
2. Sequentially numbered, opaque sealed envelopes
Examples of procedures usually considered inadequate includes :
1. Assignment of envelopes without appropriate safeguards for example – unsealed, non opaque or not sequentially numbered
Internal validity
Internal validity gives the information about the study – whether the study was done well (methodology) and whether the findings are valid.
External validity
Example
We carry out a study in one city and find that a new therapy is better than a current therapy, we would like to say that the new therapy is better for the disease regardless of where the patients are treated and not just for patients in that city.
Guidance on the ethical conduct of clinical research
Clinical research includes certain guidelines which need to be followed. They are :
I. NUREMBERG CODE
II. DECLARATION OF HELSINKI 1964
I. NUREMBERG CODE
The Nuremberg Code is a set of research ethics principles for human experimentation set as a result of the Subsequent Nuremberg Trials at the end of the Second World War.
The voluntary consent of the human subject is absolutely essential.
The experiment should be such as to yield fruitful results for the good of society, unprocurable by other methods or means of study and not random and unnecessary in nature.
The experiment should be so designed and based on the results of animal experimentation and a knowledge of the natural history of the disease or other problem under study that the anticipated results will justify the performance of the experiment.
The experiment should be so conducted as to avoid all unnecessary physical and mental suffering and injury. No experiment should be conducted where there is a prior reason to believe that death or disabling injury will occur; except in those experiments where the experimental physicians also serve as subjects.
The degree of risk to be taken should never exceed that determined by the humanitarian importance of the problem to be solved by the experiment.
Proper preparations should be made and adequate facilities provided to protect the experimental subject against even remote possibilities of injury, disability, or death.
The experiment should be conducted only by scientifically qualified persons. The highest degree of skill and care should be required through all stages of the experiment of those who conduct or engage in the experiment.
During the course of the experiment the human subject should be at liberty to bring the experiment to an end if he has reached the physical or mental state where continuation of the experiment seems to him to be impossible.
II. DECLARATION OF HELSINKI - 1964
The Declaration of Helsinki is a set of ethical principles regarding human experimentation developed for the medical community by the World Medical Association (WMA). It is widely regarded as the cornerstone document of human research ethics.
The Declaration was originally adopted in June 1964 in Helsinki, Finland.
Basic principles
The fundamental principle is respect for the individual (Article 8) their right to self determination and the right to make informed decisions (Articles 20, 21 and 22) regarding participation in research, both initially and during the course of the research.
The investigator's duty is solely to the patient (Articles 2, 3 and 10) or volunteer (Articles 16, 18), and while there is always a need for research (Article 6) the subject's welfare must always take precedence over the interests of science and society (Article 5).
Ethical considerations must always take precedence over laws and regulations (Article 9).
The recognition of the increased vulnerability of individuals and groups calls for special vigilance (Article 8).
VIII. STUDIES ON RCTs
1. In a study conducted by Arruda et al – The effect of 5% fluoride varnish application on caries among school children in rural Brazil was assessed.
It was a Randomized Controlled Trial
(Source : Effect of 5% fluoride varnish application on caries among school children in rural Brazil : A randomized controlled trial Community Dent Oral Epidemiol 2012; 40: 267–276)
Study Design
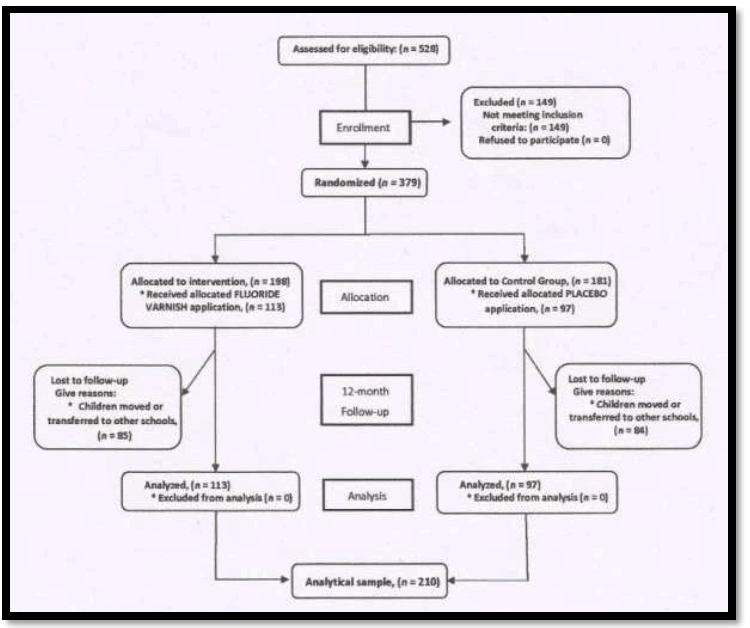
Results
The efficacy of fluoride varnish application on caries prevention was reported as a preventive fraction (PF). Crude caries increments of decayed and filled surfaces (DFS) were compared between fluoride varnish and placebo groups.At the baseline examination, the children in the treatment and control groups presented on average 6.2 and 5.6 DFS, respectively (P < 0.001).
After 12 months of follow-up, the children in the varnish group showed significantly lower DFS increments than did children in the control group (10.8 versus 13.3; P < 0.007), with PF of 40% (95% CI: 34.3–45.7%; P < 0.0001).
2. In a study conducted by Mohebbi et al - the impact of educational interventions on sugary snacking in infants and toddlers in Tehran, Iran was assessed.
It was a Community Randomized Controlled Trial
(Source : A community-randomized controlled trial against sugary snacking among infants and Toddlers Community Dent Oral Epidemiol 2012; 40 (Suppl. 1): 44–49).
Study design
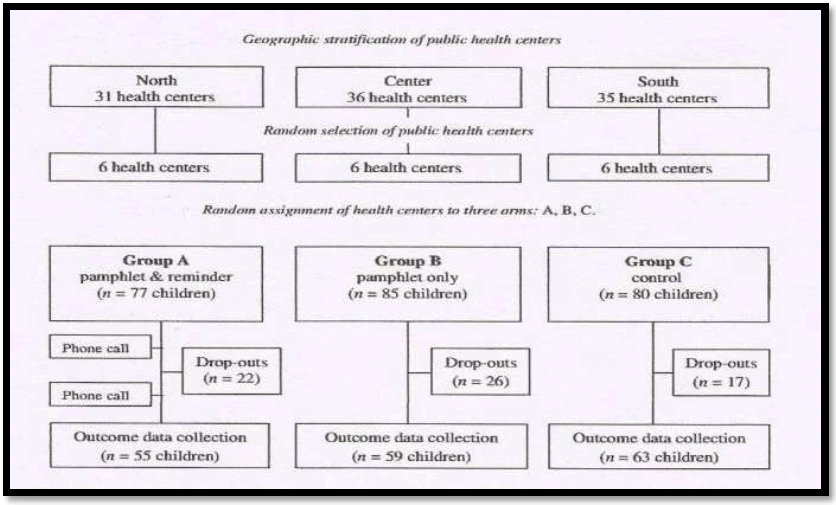
Results
In all groups, the child’s and mother’s snacking at baseline were correlated (r = 0.4). A positive outcome was found for 62% of the children in group A and for 49% and 32% in groups B and C respectively. In group A, a reduction in the children’s snacking frequency was found despite their residential area (P < 0.05). Controlling for intervention effects, the logistic regression model showed that residential area was unrelated to the positive outcome.
IX. Study Designs
Some of the study designs of controlled trials are :
I. CONCURRENT PARALLEL STUDY DESIGN
II. CROSS – OVER TYPE OF STUDY DESIGN
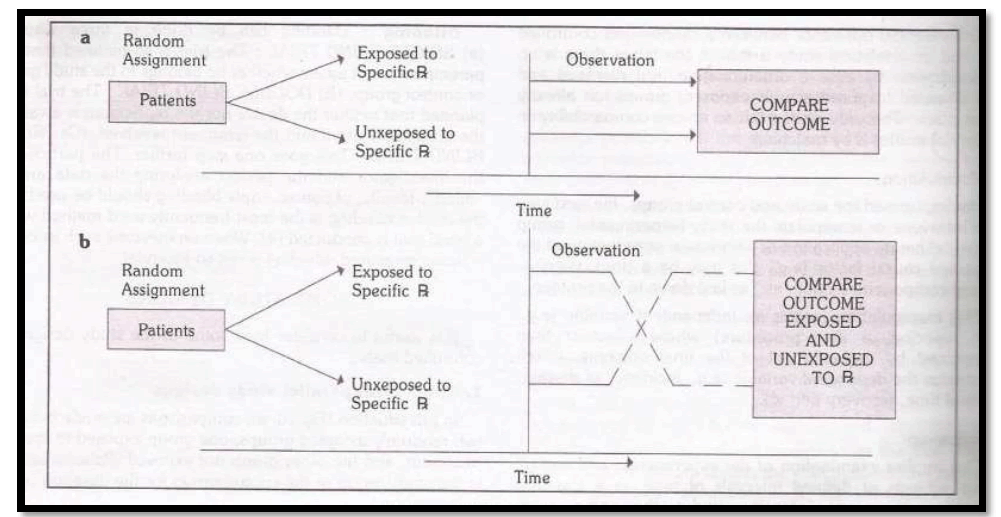
I. Concurrent study design
In this type of study design, comparisons are made between two randomly assigned groups, one group exposed to specific treatment and the other group not exposed. Patients remain in the study and control group for the duration of the investigation.
II. Cross over type of study design
With this type of study design, each patient serves as his own control. As before, the patients are randomly assigned to a study group and control group. The study group receives the treatment under consideration. The control group receives some alternate form of active treatment or a placebo. The two groups are observed over time. Then, the patients in each group are taken off their medication or placebo to allow for the elimination of the medication from the body and for the possibility of any “carryover” effects as shown in fig above. After this period of medication, the two groups are switched. Those who received the treatment under study are changed to the control group therapy or placebo and vice versa.
X. NON RANDOMIZED TRIALS
In non randomized trials, there is no randomization and the degree of comparability will be low and the chances of a spurious result higher than where randomization had taken place. A few examples are explained below.
Examples of non randomized trials
1. Uncontrolled trials
2. Natural experiments
3. Before and after comparison studies
1. Uncontrolled trials
These are the trials with no comparison group.They are useful in evaluating specific therapy to know the value of particular disease, to determine an appropriate dose of drug and to investigate adverse effects of drugs.
Example for uncontrolled trials
There were no randomized controlled studies of the benefits of Pap test for cervical cancer when it was introduced in 1920s. But, today there is indirect epidemiological evidence.
2. Natural experiments Example
A major earthquake in ATHENS in 1981 provided a “natural experiment” to epidemiologists who studied THE EFFECTS OF ACUTE STRESS ON CARDIOVASCULAR MORTALITY.
3. Before and after comparison studies
They are also the community trials. They have two groups. They are :
I. Before and after comparison studies WITHOUT control
II. Before and after comparison studies WITH control
A. Before and after comparison studies without control
These studies centre round comparing the incidence of disease before and after introduction of a preventive measure .The events which took place prior to the use of the new preventive measure are used as a standard for comparison.In other words, the experiment serves as its own control.This eliminates virtually all group differences.
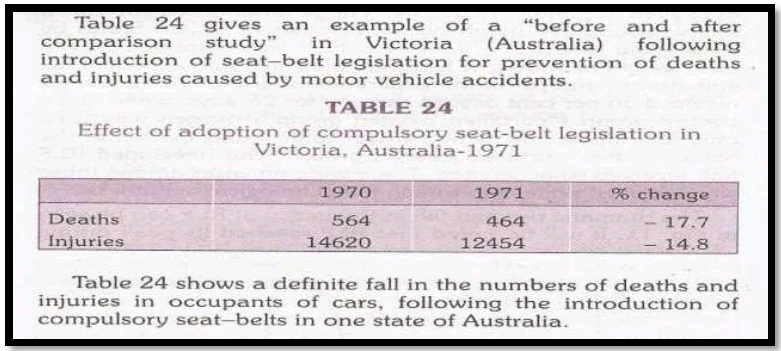
B. Before and after comparison studies with control
In the absence of a control group, comparison between observations before and after the use of new procedure may be misleading. If preventive programme is to be applied to an entire community, epidemiologist would select another community as similar as possible, particularly with respect to frequency and characteristics of the disease to be prevented.
Experimental designs
Randomized Controlled Clinical Trials (RCCTs)
They are used to test therapeutic interventions in ill persons.Study population consists of the intervention group and the control group.
RCCTs are considered the “GOLD STANDARD” for studying interventions because of their ability to minimize bias in the information obtained from the study subjects. Nevertheless, they do not entirely eliminate bias and they pose some challenges and ethical dilemmas for investigators.
Randomized Controlled Field Trials ( RCFTs)
They are usually are done to test the preventive interventionsin well persons in the community. Examples involve :
– Trials involving vaccines to prevent paralytic poliomyelitis
–Trials of aspirin to reduce cardiovascular disease
Design of Randomized Controlled Clinical Trials and Randomized Controlled Field Trials
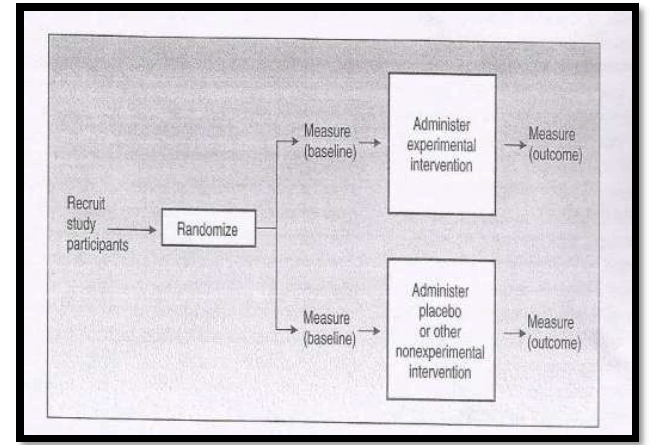
The study participants are randomly assigned into two groups. One group where experimental intervention is given and the other group with placebo. The baseline information of both the groups will be obtained. The outcome measure will be obtained in both the groups.
XI. Advantages of experimental study designs
1. Researcher has got the ‘control’ over the intervention and over which subjects receive any intervention
2. Results are ensured
3. Reliable and well-respected research design
4. Individual factors can be identified
XII. Disadvantages of experimental study designs
1. Problems in dealing with multiple causation, isolating individual factors may over-simplify complex issues
2. Ethical issues
XIII. Difficulties in experimental designs
1) Problems in setting the experiment
2) Problems in getting cooperation
3) Difficulties in establishing control
4) Time consuming
5) Expensive
6) Human behaviour is very complex and cannot be fully studied using experimental designs
XIV. CONSORT (Consolidated Standards of Reporting Trials) GUIDELINES
Randomised controlled trials, when appropriately designed, conducted and reported represent the gold standard in evaluating health-care interventions. However, randomised trials can yield biased results if they lack appropriate methodology
Consort guidelines are used to assess a trial accurately.Inadequate reporting had led to the development of the original CONSORT (Consolidated Standards of Reporting Trials) statement in 1965 and its revision 5 years later. (Source : CONSORT 2010 Statement : Updated guidelines for reporting parallel group randomised trials Kenneth F Schulz, Douglas G Altman, David Moher, for the CONSORT Group).
Ethical problems with Randomized Controlled Trial (RCT)
Ethics of Clinical Research
1. Ethical principles and Norms
2. Role of Institute Review Board (IRB)
3. Ethical issues
✓ Balance of risk and benefit
✓ Informed Consent
✓ Selection of subjects
✓Compensation for research related injury
✓ Confidentiality and Information security
✓ Research integrity
✓ Ethical problems with Randomized Trials
✓ Use of animal, human tissue and biological sample
Unique ethical issues
✓Equipoise : ethical justification of RCT
✓ Physician as clinical investigator
✓ Randomization and Blinding
✓ Preliminary data and emerging trends
✓ Placebo and/or “Best current” control treatment
✓ Continuation of treatment after trial end.
Equipoise
Ethical justification of RCT: “An honest or bona fide null hypothesis”, also referred to as equipoise. Clinical Equipoise exists if: In comparing treatment A and B
1. The clinical community agrees there is no convincing evidence that A is better or less toxic than B
2. There is no superior therapy C, unless good reason exists to reject C
Hence, doubt about which treatment is superior justifies giving subjects an equal chance to get either one; no one is being assigned to an inferior treatment.
[ Source : www.crc.gov.my ]
Summary
There are different epidemiological methods such as Descriptive, Analytical and Experimental. Experimental research is a study in which an investigator manipulates or controls one or more independent variables and observes the effect on dependent variables. Types of experimental studies – Randomized controlled Trials and Non-randomized Controlled Trials.There are various steps involved in randomized controlled trials. There are types of randomized controlled trials such as clinical trials, preventive trials, risk factor trials,cessation experiments, trial of etiological agents, evaluation of health services and community Intervention Trials(CITs).Further, there are types of Non-Randomized Controlled trials. There are Concurrent parallel study design and Cross - over type of study designs.There are various advantages and disadvantages of experimental studies. There are guidelines to be followed when conducting RCTs such as CONSORT guidelines.
Conclusion
❏ In experimental or intervention studies - conditions in which study is carried out are under the direct control of the investigator. Thus, experimental studies involve some action, intervention or manipulation.
❏ Randomized controlled trials provide the best evidence of the efficacy of medical interventions but they are not immune to bias.
References
- Park K. Textbook of Preventive and Social Medicine. 21st edition pg.no. 76-82.
- Peter S. Essentials of Preventive and Community Dentistry. 4th edition pg.no.42-80.
- Kothari C.R. Research Methodology. 2nd edition 2004 pg.no.39-52.
- Daly B. Essentials of Dental Public Health.1st edition 2002 pg.no.65-80.
- Pine C. Community Oral Health. 2nd edition 2007 pg.no.115-135.
- Mason J. Concepts in Dental Public Health. 2nd edition 2010 pg no. 178-191.
- Gordis L. Epidemiology 4th edition 2009 pg.no.131-164.
- Fris R.H. Epidemiology for Public Health Practice.4th edition 2009 pg.no. 67-89.
- Detels R, Beaglehole R. Oxford Textbook of Public Health. 5th edition 2007 pg.no. 526-540.
- Jekel J.F, Katz D.L. Epidemiology Biostatistics and Preventive Medicin.e 3rd edition 2007 pg.no. 83-86.
- Burt B.A, Eklund S.A. Dentistry Dental Practice and the Community. 6th edition 2005 pg.no. 177-182.
- Fletcher R.W. Clinical Epidemiology – The Essentials. 4th edition 2005 pg.no.128-143.
- www.holah.karoo.net/evaluationofexperiment.html.
Citation: Suvarna V Biradar, Divya S, Experimental Epidemiology – A Review, Asian Journal of Pharmaceutical Technology & Innovation, 6 (27); 10-35, 2018. www.asianpharmtech.com.
