Short Commentary- Asian Journal of Pharmaceutical Technology and Innovation(2018)
Review on Various Analytical Method for Quantitative Determination of Etophylline and Theophylline in Bulk and in Different Dosage Forms
Arina Macwan*, Vanita Marvaniya and Pragnesh PataniArina Macwan, Department of Quality Assurance, A-One Pharmacy College, Ahmedabad, Gujarat, India, Tel: 91- 9496783021, Email: arinamacwan@gmail.com
Received: 02-Apr-2018 Accepted Date: Apr 16, 2018 ; Published: 23-Apr-2018
Abstract
Theophylline is used to treat lung disease and COPD (Bronchitis, emphysema). Etophylline is derivative of Theophylline, used as antiasthmatic agent. It is adenosine antagonist that relaxes muscles of bronchi. They are under the class of drug Xanthine. These medicines need to be used regularly for relief of asthmatic symptoms. Various analytical methods are reported for estimation and stability of Theophylline and Etophylline in bulk and in different Formulation. A comprehensive Literature review is prepared for Theophylline and Etophylline.
Keywords
Theophylline, Etophylline, RP-HPLC, Spectrophotometric
Introduction
Etophylline (1-5)
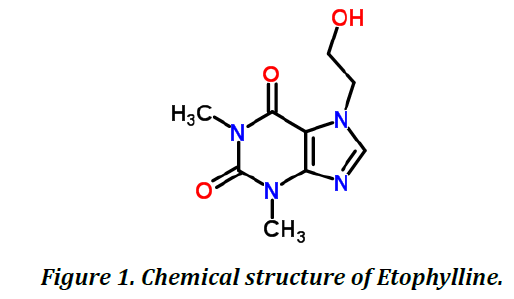
Etophylline is chemical derivative of Theophylline, belongs to class Xanthines (Figure 1). It is indicated in Asthama, Chronic obstructive pulmonary disease, chronic bronchitis and emphesema (Table 1).
| Sr. No. | Parameters | Description |
|---|---|---|
| 1 | Category | Bronchodilator Agent |
| 2 | Structure[2] | 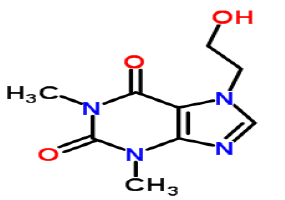 |
| 3 | Chemical Formula | C9H12N4O3 |
| 4 | IUPAC Name | 7-(2-Hydroxyethyl) 1,3-dimethyl purine-2,6-dione |
| 5 | Molecular Weight | 224.22 g/mol |
| 6 | Characteristic | White Crystalline powder or crystals |
| 7 | Solubility | Freely soluble in water and soluble in methanol. Moderately soluble in Ethanol, Sparingly soluble in chloroform, Practically insoluble in ether |
| 8 | Log P | 1.24 |
| 9 | Melting Range | 160-165°C |
| 10 | Storage | Store at 2 to 8°C |
| 11 | CAS No. | 519379 |
| 12 | Indication | Chronic obstructive bronchoneumopathy, Asthma |
Table 1: Drug profile of etophylline.
Official Methods for Etophylline
1. In European Pharmacopeia 6.0 published by EDQMH, the detection of Etophylline is by HPTLC method where mobile phase Ammonia, Ethanol and Chloroform (1:10:90, v/v/v); plate HF 254, Wavelength 254 nm.
2. The Second Method found in European Pharmacopeia 6.0 Potentiometric method where Titrant 0.1 M Perchloric acid is while Titrate 0.2 g Etofylline in 50 ml Acetic anhydride.
Reported Method for Etophylline
Various analytical methods are reported for Etophylline in bulk and in pharmaceutical formulation.
1.The Derivative Spectrophotometric method solution methanol wavelength 273 nm.
2. The HPTLC method is having mobile phase of Acetone, Methanol and Formic acid (9:3:0.01, v/v/v); Plate F275, having wavelength 275 nm.
3. The HPLC method was having mobile phase, Column is Waters Spherisorbs of dimensions (250 mm × 4.6 mm, 5 μ); Mobile phase is 0.02 M Ammonium Acetate buffer (Methanol: Acetonoitrile [70:30]) pH4.5 adjusted flow rate is 1 ml/min of wavelength 275 nm.
Theophylline (4,6-8)
Theophylline is used to treat respiratory disease like chronic obstructive pulmonary disease (COPD) and Asthma; chemically it is 13-dimethylexanthine. It is naturally found in Tea and Cocoa, as the drugs belongs to xanthine family are naturally found. A small amount of Theophylline is produced in liver by metabolizing Caffeine.
Theophylline is official in The United States Pharmacopeial Convention (USP 32-NF 27) and European pharmacopoeia (Ph Eur 6.0).
Official Methods of Theophylline (9-16)
1. The RP-HPLC method was found in USP 32-NF-27for theophylline having mobile phase 0.05 M Sodium Acetate trihydrate+0.01% Glacial Acetic Acid: Acetonitrile (30:70) having Colum packing of L1 with the dimensions of (300 mm × 4.0mm, 5 μ), flow rate 1 ml/min. Wavelength 280 nm.
2. In European Pharmacopeia 6.0 RP-HPLC method has been described having mobile phase Acetonitrile: 10 mM Sodium Acetate pH was adjusted with Glacial Acetic Acid, Column packing L1 (250 mm × 4.00 mm, 7 μ), flowrate of 2 ml/min and wavelength was 272.
Reported Method of Theophylline
1. The different journals has reported articles on theophylline, one of them is by Derivative Spectrophotometric Method where wavelength was 244 nm and the range of 4-12 μg/ml.
2. The other reported method is RP-HPLC where mobile phase was found to be of 10 mM Ammonium Acetate buffer, Acetonitrile and Methanol where pH 6.0 was adjusted and the ratio was found to be (90:5:5, v/v/v), flow rate is 1 ml/min of wavelength 272 nm, column of Purosphere C18 was selected having dimensions of (250 mm × 4 mm, 5 μm).
3. The other reported method is Ion-Pair Liquid Chromatography where selection of mobile phase of Acetic Acid (1%) and methanol along with buffer 3 mM Sodium-1-Octane Sulphonate as ion pairing agent was used Column of Spherisorb C18 (250 mm × 4.6 mm, 10 μm) having flow rate of 1 ml/min and wavelength was selected as 277 nm.
4. HPLC method was found to be approaching having mobile phase of Acetonitrile and Water in the ratio of (25:75, v/v) column of Inertsil C18 (250 mm × 4.6 mm, 5 μm) was used having flow rate of 1 ml/min and wavelength of 275 nm.
5. One of the UV Spectrometry methods was found where selection of wavelength can be done. The author selected wavelength of 271 nm.
6. One of the UV Spectrometry methods was referred for solvent selection of methanol and water with the ratio of (1:1). Where wavelength hereby was selected as 273 nm.
7. HPLC method was convincing having mobile phase as Acetonitrile and 50 mM Sodium Acetate Buffer having ratio of (15:85, v/v) where pH was adjusted to pH 6.0 with dilute Hydrochloric Acid, Column of Hi-Q-Sil C18 (250 mm × 4.6 mm, 5 μm) dimensions where selected and the flow rate was found to be 1 ml/min wavelength of 270 nm.
8. The other HPTLC method having mobile phase of Chloroform and Acetone (1:1, v/v) and plate of F254 was selected for the detection
9. The mobile phase of the HPLC method was found to be methanol and 10 mM Tetrabutyl Ammonium Hydrogen Sulphate with the ratio of (50:50, v/v), where column of Phenomenax was selected of dimensions (250 mm × 4.6 mm, 5 μm), flow rate of 1 ml/min and wavelength was selected 274 nm.
10.Reported Method for Theophylline and Etophylline in Combination Here the reported method the mobile phase and column has been changed but the analytical technique remains the same, now the mobile phase consist of Acteonitrile and Phosphate buffer with the ratio of (65:35, v/v) where pH 4.2 was adjusted with Triethylene Amine, Column Zorbax ODS was selected the dimensions categorized as (250 mm × 4.6 mm, 5 μm) having wavelength of 210 nm and flow rate 1.5 ml/min for the detection.
Reported Method for Theophylline and Etophylline in Combination
1. There are number of methods reported for the both drugs individually but in combination there is limitation.
2.Least number of methods found where HPLC and UV Spectroscopy is the main.
3.The HPLC methods consist of different mobile phase and column.
4.Through the study of these articles selection of wavelength can be easy and accurate.
5.Not only wavelength can also give brief idea of mobile phase also.
6. From the detailed study of literature it has been found that the combination having developed analytical method is least and mostly found in parenteral dosage form and Tablet dosage form.
7. Syrup can be considered to be the newly developed dosage form and can be moved forward for the analytical development.
Conclusion
The present review depicts the reported spectrophotometric and chromatographic methods which had developed and validated for the estimation of Etophylline and Theophylline. According to the literature review it has been concluded that the different spectrophotometric and chromatographic methods are available for the estimation of Etophylline and Theophylline in the bulk drug as well as in combination in different dosage form. These all methods are accurate, precise, reproducible, and robust. Most of the methods are of RP-HPLC and of UV Spectrophotometry as these methods provides best available reliability, repeatability, analysis time and sensitivity.
References
- National Formulary of India, 5th Edn, 2016.
- British Pharmacopoeia, Monograph: Medicinal and pharmaceutical science: Etofylline, 1; 2009.
- Pharmacopeia European Monograph, European Directorate for Quality of Medicines & Healthcare, 6; 1907.
- Indian Pharmacopeia, Ministry of Health and Family Welfare, 1; 97, 2010.
- Dave HN, Mashru RC, Patel AK, Thin layer chromatography method for the determination of ternary mixture containing salbutamol sulphate, bromhexine hydrochloride and etofylline, J Pharm Sci Res, 2 (2); 390-394, 2010.
- USP32–NF27, Pharmacopeial forum, 29: 1586.
- British Pharmacopoeia, Monograph: Medicinal and pharmaceutical science, Theophylline, 1; 2009.
- Pharmacopeia European, Monograph 6.0. European Directorate for quality of medicines & Healthcare, 2017.
- Hasegawa T, Ishi T, Nagata T, Takahashi K, Saijo M, Rapid determination of theophylline, theobromine and caffeine in dietary supplements containing guarana by UPLC, Shokuhin Eiseigaku Zasshi,50 (6); 304-310, 2009.
- Patel RB, Parmar RR, Patel VM, Shah DA, Simultaneous estimation of montelukast sodium and theophylline in pharmacuetical dosage form by UV spectrophotometric method, International Journal of Institutional Pharmacy and Life Sciences, 2 (2); 2012.
- Layloff TP, Flinn PE, Juhl YH, A simple, inexpensive thin layer chromatography method for the analysis of theophylline tablets, Bull World Health Organ, 67 (5); 555-559, 1989.
- Sultan M, Simultaneous HPLC determination and validation of amphetamine, methamphetamine, caffeine, paracetamol and theophylline in illicit seized tablets, Int J Pharm Pharm Sci, 6 (4); 2014.
- Panda SS, Varaha BV, Kumar R, Mohanta G, Stability-indicating RP-HPLC method for simultaneous estimation of levosalbutamol sulfate and theophylline in combined dosage form. Braz J Pharm Sci, 49 (3); 475-490, 2013.
- Maithani M, Singh R, Development and validation of a stability-indicating HPLC method for the simultaneous determination of salbutamol sulphate and theophylline in pharmaceutical dosage forms, J Anal Bioanal Techniques, 2 (1); 116, 2009.
- Sawant RL, Bharat AV, Tanpure KD, Jadhav KA, A new RP-HPLC method development for simultaneous estimation of salbutamol sulphate, theophylline and furosemide, Int J Pharma Sci Res, 6 (4); 2015.
- Abdelwahab NS, Determination of ambroxol hydrochloride, guaifenesin and theophylline in their ternary mixtures and in the presence of excipients in different pharmaceutical dosage forms, J AOAC Int, 95 (6); 1629-1638, 2012.
Citation: Arina Macwan, Vanita Marvaniya, Pragnesh Patani, Review on Various Analytical Method for Quantitative Determination of Etophylline and Theophylline in Bulk and in Different Dosage Forms, Asian Journal of Pharmaceutical Technology & Innovation, 6 (28); 1-10, 2018.