Research Article- Asian Journal of Pharmaceutical Technology and Innovation(2017)
Formulation of an Optimised Glimepiride Compression Coated Tablets for Chronotherapeutic Drug Delivery
Airemwen C.O*, Uhumwangho M.U and Obakpolor S.ODr. Airemwen C.O, Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, University of Benin, P.M.B 1154, Benin-City, Nigeria, Tel: +234 8133737933, Email: collins.airemwen@uniben.edu
Received: 10-Oct-2017 Accepted Date: Oct 18, 2017 ; Published: 20-Oct-2017
Abstract
Background: Chronopharmaceutics is an aspect of pharmaceutics devoted to the formulation of drug delivery systems that release their active ingredients at a rhythm that ideally matches the physiological requirement of a particular disease condition. Objective: The aim of the present study is to formulate an optimized coated glimepiride time release tablets (CGTRT) containing glimepiride, a third generation sulphonylurea in the inner core for chronotherapeutic drug delivery system. Method: Glimepiride core tablets (GCT) containing Anacardium occidentale gum (10 %w/w) as binder and maize starch as disintegrant (10 %w/w) were prepared at compression pressure of 30 unit on the arbitrary load scale. CGTRT containing Anacardium occidentale gum powder with sodium starch glycolate at varying concentration (0.5%, 1%, 2%, 4%, and 6%w/w) were also prepared at 30 unit on the arbitrary load scale. The parameters determined were hardness, friability, drug content, disintegration time and in vitro drug release profile. Result: The hardness value of the core tablet and market formulation was > 4.1 Kpa while their friability values were 1.7% (core) and 1.2% (market formulation). The core tablet and market formulation disintegrated in 7min and 6min respectively, while their drug content was > 97%. The hardness and friability values of the CGTRT were > 12 KPa and < 0.59% respectively. All CGTRT displayed different lag time and this was dependent on the concentration of the sodium starch glycolate incorporated into the CGTRT. The drug content of the formulations was > 94%. Conclusion: CGTRT1 with a lag time of 6 h before release of glimepiride was taken as the optimized formulation..
Keywords
Glimepiride, Anarcadium occidentale gum, Glimepiride core tabletIntroduction
Diabetes mellitus is defined as a metabolic disorder of multiple aetiology characterized by chronic hyperglycaemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action, or both [1]. Cardinal symptoms of hyperglycaemia include polyuria (frequent urination), polydipsia (increased thirst), and polyphagia (increased hunger). Chronic hyperglycaemia is associated with microvascular and macrovascular complications that can lead to visual impairment, renal failure, retinopathy, nerve damage, amputation, stroke and heart disease [2]. There are three main types of diabetes mellitus: Type 1 diabetes mellitus - this result from failure of the pancreas to produce enough insulin. It could be due to an autoimmune destruction of pancreatic β-cells in the islet of Langerhans. This form of diabetes mellitus was previously referred to as insulin-dependent diabetes mellitus (IDDM) or juvenile diabetes.
Type 2 diabetes mellitus- this begins with insulin resistance, a condition in which cells fail to respond to insulin properly. Lack of insulin may develop as the disease progresses. This form was previously referred to as non-insulin dependent diabetes mellitus or adult-onset diabetes mellitus. The primary cause is excessive body weight and inadequate exercise.
Gestational diabetes- this occurs in pregnant women without previous history of diabetes. It is characterized by elevated blood sugar levels during gestation [1].
Plasma glucose concentrations show a 24 h rhythm regulated by the biological clock in the suprachiasmatic nucleus, with the highest concentrations seen towards the beginning of the activity period and declining concentrations in the dark period [2]. Increased daytime glucose tolerance is due to increased pancreatic β-cells insulin secretion, increased insulin receptors availability and sensitivity. Impaired insulin sensitivity is a result of reduced access and sensitivity of insulin receptors, which reduces peripheral glucose uptake [3].
Chronotherapy is defined as a medical treatment administered according to a schedule that corresponds to a person's daily, monthly, seasonal, or yearly biological clock or the treatment of a sleep disorder by altering an individual's sleeping and waking times and resetting his or her biological clock [4,5]. While, chronopharmacology studies the effects/side effects of drugs upon temporal changes in biological functions or symptoms of a disease as well as drug effects as a function of biologic timing [6]. Drugs used in the management of asthma, hypertension and diabetes have been investigated for chronotherapeutics because these symptoms follow circadian rhythms [7]. Hence, the rationale of this research is to formulate an optimized chronotherapeutic drug delivery system (ChrDDS) of glimepiride using Anarcadium occidentale gums in different ratios with a predetermined lag time.
Cashew gum is gotten from the stem bark of Anacardium occidentale L. (family: Anacardiaceae) as exudates. The tree grows freely in many tropical and subtropical countries8 and its fruits and seeds are consumed locally as it is a source of protein, carbohydrate, fat and vitamins. The bark of cashew exudes a gummy material called cashew gum. It is a complex polysaccharide, comprising 5% arabinose, 70% galactose, 1% mannose, 4% rhamnose, and 6% glucuronic acid [9]. Cashew gum has been previously used in the formulation of metronidazole tablets, and has been shown to have good binding properties [10,11]. However, its use as a binder in ChrDDS of glimepiride has not been investigated.
Glimepiride is a third generation sulfonylurea compound with potent hypoglycaemic activity. It enhances the release of insulin from pancreatic beta cells; and increases sensitivity of intracellular insulin receptors. Glimepiride also increases intrinsic peroxisome proliferator receptor γ (PPR-γ) activity. It is used in the treatment of type 2 diabetes mellitus [12]. It’s completely absorbed (100%) from the gastrointestinal tract; metabolized by Cytochrome P450 enzyme system of the liver. It has a half-life of 5-8 h; excretion is via the urine (60%) and faeces (40%). Time to attain peak plasma concentration is 2-4 h [13]. Glimepiride is classified under class II of the biopharmaceutical scheme [14].
The aim of the study was to formulate a CGTRT based on chronopharmaceutical technique for the management of Type II diabetes mellitus using glimepiride as a model drug. The aim was to obtain a lag time of 6 h, i.e. the tablet is to be taken at bed time (8 pm) and it is expected to release its drug after a period of 6 h 1.e. at 2 am Literatures show that the time to reach peak plasma concentration for glimepiride is 2- 4 h after oral administration [12]; meaning, if CGTRT1 should be administered to a patient, it would take 8-10 h for glimepiride to achieve peak plasma concentration. Thus if the formulation is administered at 8 pm, peak plasma concentration would be reached between 4 am and 6am; just about the time when symptoms of dawn phenomenon observed in diabetic patients are most prevalent.
Materials and Method
Glimepiride was a gift sample from Healthy Life Pharma PVT. Ltd, India. Maize starch powder (BDH, Chemical, Poole, UK) was incorporated as a disintegrant in the core tablet. Anacardium gum was used as the binder and talc was served as the glidant at a concentration of 1% w/w. AO gums were extracted by methods described earlier [15]. All other chemicals were of analytical grades.
Method
Extraction of Anacardium occidentale gum
The extraction of Anacardium occidentale gum was carried out using a known method by Kwabena et al., 15] with some modifications. The dried exudates were cleaned by hand picking barks and other extraneous materials. 100 g of the dried gum was milled in a blender and dissolved in 200 ml of distilled water and allowed to stand for 24 h with intermittent stirring. The resulting mixture was filtered using a muslin bag to remove insoluble debris or impurities. The filtrate was purified by precipitating the gum out with 96% ethanol. The precipitated gum was dried in a hot air oven at 50°C for 48 h. The dry flakes were pulverized using a mortar and pestle, weighed and then stored in an air tight container
Preparation of Glimepiride core tablet (GCT)
The wet granulation technique was used in the preparation of the granules for the core tablets using Anacardium occidentale gum as binder. 10% w/w of binder was mixed with sufficient amount of water in a mortar to form the granulating fluid and it was used to triturate the appropriate amount of glimepiride and lactose in the mortar. Half of the required amount of 10%w/w maize starch was then added intragranularly and mixed intimately to produce a damp mass which was passed through a sieve of 850 μm aperture, then oven dried at 60o C for 30 min. The dried granules were subsequently passed through a 710 μm aperture sieve. The remaining half of 10%w/w maize starch (extra-granularly) and 1%w/w talc were added to the dried granules. The resulting granules were compressed into tablets using the single punch tableting machine (Type P3, No: 5L 182, Manesty machines LTD, Liverpool, England) under a compression pressure of 30 unit on the arbitrary load scale after all the necessary flow properties were done. The formular table is shown in Table 1.
| Ingredients | Composition |
|---|---|
| Glimepiride | 4 mg |
| Maize starch | 10% w/w |
| Anacardium occidentale gum | 10% w/w |
| Lactose | 5% w/w |
| Talc | 1% w/w |
Table 1: Composition of Glimepiride Core Tablet.
Preparation of Coated glimepiride time release tablet (CGTRT)
The binder (cashew gum) and sodium starch glycolate (SSG) were accurately weighed as required for 5 batches of 10 tablets each as shown in Table 2 and mixed intimately in a mortar; such that each tablet contained 500 mg (0.5g) of Anacardium occidentale (Cashew) gum, while the amount of sodium starch glycolate (super disintegrant) was varied. Tablets in batch 1 (CGTRT1) contained 0.5% SSG, batch 2 (CGRTRT2) contained 1% SSG, batch 3(CGTRT3) contained 2% SSG, batch 4 (CGTRT4) contained 4% SSG and batch 5 (CGTRT5) contained 6% SSG. Half of the polymer blend was placed inside the die to make a powder bed. The core tablet was placed at the centre of the polymer bed, while the remaining half of the polymer blend was filled into the die to cover the core tablet. The contents of the die were then compressed using a single punch tableting machine under a compression pressure of 30 unit on the arbitrary load scale.
| Ingredients | CGTRT1 | CGTRT2 | CGTRT3 | CGTRT4 | CGTRT5 |
|---|---|---|---|---|---|
| Anacardium occidentale gum (mg) | 500 | 500 | 500 | 500 | 500 |
| Sodium starch glycolate (mg) | 2.5 | 5 | 10 | 20 | 30 |
Table 2: Composition of coated glimepiride time release tablets (CGTRT).
Evaluation of Glimepiride Core Tablets and CCTRTs
Hardness test
Tablet hardness was determined by diametrical compression, and it refers to the pressure required to break a tablet placed on the anvil of the hardness tester. The hardness test was determined using the Mosanto hardness tester (Mosanto chemical company, Liverpool, England).
Friability test
The weight of five each of core tablets, market formulation (TEVA) and CCTRTs (1,2,3,4,5) was determined , then subjected to cascading and free fall shocks in the drum of the Roche Friabilator (Erweka, Germany) operated at 25 rpm for 4 min. Afterwards, the tablets were re-weighed after dusting off adherent particles and the percentage friability calculated using equation 1.
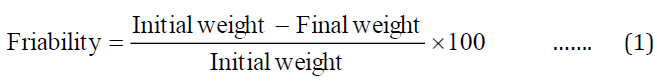
Disintegration test
Six each of core tablets and market formulation (TEVA) were individually subjected to the B.P disintegration test [16]. The disintegration medium was maintained at a temperature of 37 ± 2°C. The average value of the disintegration time was obtained.
Dissolution test
The paddle method was used. One tablet was placed in each beaker of the six station dissolution apparatus (ST7, G.B. Caleva Ltd, England) containing 900 ml of phosphate buffer (pH 7.8) maintained at a temperature of 37 ± 2°C. The paddle was rotated at 75 rpm; the test was run for 2 h for both core tablets and market formulation, and 8 h for CGTRTs (1, 2, 3, 4, 5). Aliquots of 5 ml was withdrawn at predetermined time intervals of 5, 10, 15 and 30 min; 1 and 2 h for core tablet and market formulation; 15 and 30 min; 1, 2, 4, 6 and 8 h for CGTRTs. Aliquots were withdrawn using a pipette fitted with a cotton wool plug, replaced with equal volume of drug free dissolution medium. Each sample collected was diluted 1: 100 with phosphate buffer (pH 7.8) and analyzed at a wavelength of 228 nm using UV spectrophotometer (T70 PG instruments Ltd). The amount of drug released was expressed as a percentage of drug content. The test was conducted in triplicate and the average value recorded.
Drug content determination
Three each of core tablet, market formulation and CCTRT were weighed and crush into fine powder separately. The powder equivalent to 100 mg of glimepiride was weighed, transferred to 100ml volumetric flask and made up to volume with phosphate buffer (pH 7.8). The resulting solution was filtered, 1ml volume was measured and further diluted 1:100 with phosphate buffer (pH 7.8). The absorbance of the resulting solution was measured at 228 nm using UV spectrophotometer (T70 PG instruments Ltd), and the percentage drug content calculated.
Conclusion
This study has been able to modify the lag time of coated glimepiride time release tablets of 0.25 – 6 h, when appropriate concentration of sodium starch glycolate is mixed with AO gum in the outer layer of the tablet. Formulation CCTRT1 containing 0.5 %w/w of sodium starch glycolate with a lag time of 6 h before burst release of glimepiride was taken as the optimized formulation.
This can be exploited to achieve chronopharmaceutical drug delivery system (ChrDDS) of glimepiride for the management of dawn phenomenon (early morning hyperglycaemia) in type II diabetics when SSG, AO gum and other excipients are mixed together in the right proportion.
References
- World Health Organization, Department of Noncommunicable disease Surveillance, Geneva Definition, Diagnosis and Classification of Diabetes Mellitus and its complications, A Report of WHO consultation, 1999.
- Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidermic of type 2 diabetes in youth. Diabetes Care. 1999; 22(2): 345-354.
- La Fleur SE. Daily rhythms in glucose metabolism: Suprachiasmatic nucleus output to peripheral tissue. J of neuroendocrinology. 2001; 15: 315-322.
- Sunil SA, Rao NS, Srikanth MV, Uhumwangho MU, Kumar KS, Ramana Murthy KV. Development and evaluation of a chronotherapeutic drug delivery system of torsemide. Braz J Pharm Sci. 2011; 47:593-600.
- Uhumwangho MU, Latha K, Sunil SA, Srikanth MV, Kumar KS, Ramana Murthy KV. Chronopharmaceutic Drug Delivery Systems (ChDDs)- a review. Res J Pharm Tech. 2011; 4:197-202.
- Latha K, Uhumwangho MU, Sunil SA, Srikanth MV, Ramana Murthy KV. Chronobiology and Chronotherapy of Hypertension – A Review, Int J of Health Res, 2010; 3(3): 121-131.
- Airemwen CO, Akpan EF, Uhumwangho MU. Development and evaluation of an optimised theophylline timed release tablets for chronotherapeutic drug delivery in nocturnal Asthma; Port Harcourt Med J. 2015;9:168-174.
- Miranda RL. Cashew tree bark secretion – perspectives for its use in protein isolation strategies. Open Glycoscience. 2009; 2: 16-19.
- De Paula RCM, Heatley F, Budd PM. Characterization of Anacardium occidentale exudates polysaccharide. Polym. Int., 1998; 45 (1): 27-35.
- Onunkwo GC, Okoye J. Evaluation of Anacardium occidentale gum as a binder in lactose based tablet formulations. Boll Chim Farm. 1997; 136 (9): 569-574.
- Ofori-Kwakye, Asantewa Y, Kipo SI. Physicochemical and binding properties of cashew tree gum in metronidazole tablet formulations. Int. J. Pharm. & Pharm. Sci. 2010; 2 (4): 105-109.
- Massimo MB. Glimepiride in type 2 diabetes mellitus: a review of the worldwide therapeutic experience. Clinical Therapeutics. 2003; 25, 799–816.
- Schernthaner G, Grimaldi A, Di Mario U, Drzewoski J, Kempler P, Kvapil M, Novialis A, Rottiers R, Rutten GEHM, Shaw KM. Double blind comparison of once-daily gliclazide MR and glimepiride in type 2 diabetic patients. Eur J of Clini Investigation. 2004; 34: 535-542.
- Frick A, Möller H, Wirbitzki E. Biopharmaceutical characterization of oral immediate release drug products. In vivo/in vitro comparison of phenoxy methyl penicillin potassium, glimepiride and levofloxacin. Eur. J. Pharm. & Biopharm.1998; 46, 305–311.
- Kwabena OK, Asantewaa Y, Samuel LK. Physicochemical and binding properties of cashew tree gum in metronidazole Tablet formulations. Int J Pharm Sci. 2010; 2: 105-9.
- British Pharmacopoeia. London, UK: Her Majesty’s Stationery Office: 2002; A234.
- Mohanachandran PS, Sindhumol PG, Kiran TS. Superdisntegrants: An overview, Int J of Pharm Sci. 2011; 6(1) 105-109.
Citation: Airemwen C.O, Uhumwangho M.U and Obakpolor S.O, Formulation of an Optimised Glimepiride Compression Coated Tablets for Chronotherapeutic Drug Delivery, Asian Journal of Pharmaceutical Technology & Innovation, 05 (26); 01-08, 2017.
